To prepare for the future, we need standardisation not only to reduce the chance of error and inaccuracy, but also for successful digital pathology practices and future AI developments. Advanced staining techniques are at the heart of this progress. By focusing on consistency, accuracy and validated methodologies, laboratories can adopt protocols that not only meet today's demands but also anticipate tomorrow's needs.
Recent innovations in advanced staining are aimed at simplifying workflows while enhancing diagnostic precision. These include systems that employ optimally validated antibodies, faster throughput and integration with digital pathology solutions. With these innovations, laboratories can better support the needs of both pathologists and patients by achieving reproducible and high-quality results consistently.
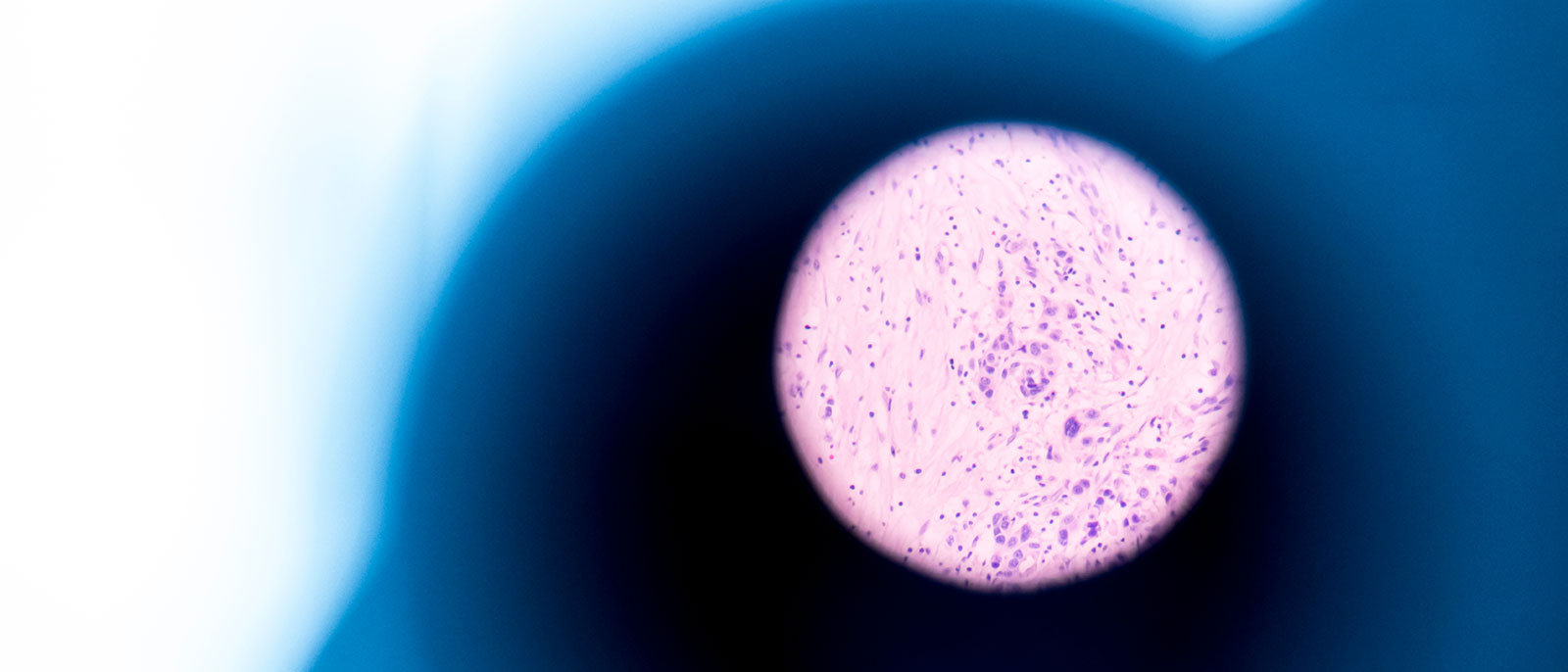
Five years ago, NordiQC concluded that, although 67% of immunohistochemistry (IHC) slides were accurate enough to make a diagnosis, one out of three slides (33%) were, and are still, insufficient¹. The study which analysed more than 30,000 IHC slides between 2003–2015, found that 30% of the staining results in the general module, and 20% in the breast cancer IHC module, were inadequate for diagnostic use.
Some reasons for this insufficiency included underdeveloped antibodies, poorly calibrated ready-to-use products, erroneous epitope retrieval – and, most importantly – delayed standardisation. Closer analysis revealed that most laboratories faced challenges with calibrating and validating IHC assays for optimal performance. In 2022, this performance was reflected on. Today, only one in five slides is insufficient for diagnosis². This jump may be attributed to factors such as access to sophisticated instrumentation for IHC, or publications that provide all stakeholders with guidelines on how to optimise IHC methods.
Regardless of the improvement, it was concluded that further IHC test accuracy and precision were required. Standardisation remains the key to perfection. By leveraging new staining systems that focus solely on achieving reproducible, optimal results, laboratories can further align with IVDR compliance and prepare for emerging trends in digital pathology and artificial intelligence.
EMWEB0022EN